Biopharmaceuticals (biological medicines) have become fundamental in modern medicine. Unlike traditional drugs synthesized chemically, biopharmaceuticals are derived from living organisms or biotechnological processes. Below, we answer several frequently asked questions on the topic.
What does biopharmaceuticals mean?
A biopharmaceutical is any pharmaceutical product whose active ingredient originates from a biological source (e.g., cells or tissues of living organisms). In other words, they are medicines derived from living beings or created through biotechnology.
These products include a wide range of substances such as therapeutic proteins (antibodies, hormones), vaccines, blood components (plasma, clotting factors), living cells used in cell therapies, and even genes used in gene therapy. Unlike conventional drugs (small chemical molecules), biopharmaceuticals are usually large and complex molecules (such as proteins or nucleic acids), or even entire cells. Therefore, they are not obtained through chemical synthesis but produced using biological systems (cell cultures, bacteria, yeasts, etc.). In summary, the term biopharmaceutical encompasses any medicine of biological origin, from a simple vaccine to advanced cell or gene therapies.
What is biopharmaceutical classification system?
The Biopharmaceutical Classification System (BCS) is a tool used in pharmacology to classify drugs based on their solubility and permeability. Proposed in 1995 by scientist Gordon Amidon, it predicts how a drug will be absorbed in the body when taken orally. This classification helps determine whether a drug will dissolve easily in the digestive tract and whether it can cross biological membranes to enter the bloodstream. The BCS defines four classes of drugs:
- Class I: High permeability and high solubility – These drugs are easily absorbed; a typical example is propranolol.
- Class II: High permeability and low solubility – They absorb well once dissolved, but have poor solubility; e.g., ibuprofen.
- Class III: Low permeability and high solubility – They dissolve easily but have difficulty crossing membranes; e.g., ranitidine.
- Class IV: Low permeability and low solubility – They have both poor dissolution and poor absorption; e.g., chlorothiazide.

What is the use of this classification?
Mainly, for regulatory and pharmaceutical development purposes. Knowing the BCS class of a drug allows for decisions such as whether a generic version can demonstrate efficacy with just lab dissolution tests rather than expensive human trials (called biowaivers). In short, the BCS links the physicochemical properties of a drug with its behavior in the body, aiding drug development and bioequivalence evaluation
What is a biopharmaceutical company?
A biopharmaceutical company is a pharmaceutical firm specialized in the research, development, and production of biological medicines (biopharmaceuticals) using biotechnological methods. Simply put, these companies use living organisms or their components (cells, genes, proteins) to create medicines. From an industrial standpoint, the biopharmaceutical industry is a subset of the broader pharmaceutical industry.
A company is considered biopharmaceutical when it employs biotechnology in its techniques and manufacturing processes. This includes both emerging biotech startups and large pharmaceutical companies with divisions dedicated to biologics.
They typically engage in genetic engineering to develop new products (e.g., inserting human genes into bacteria or mammalian cells to produce a therapeutic protein like insulin), cultivate these cells at scale, purify the resulting product, and conduct clinical trials to demonstrate safety and efficacy.

Is biopharma the same as pharma?
In everyday language, “pharma” refers to the pharmaceutical industry in general, while “biopharma” specifically refers to the biotechnological drug segment. They are not exactly the same, though they are closely related. As explained, biopharma is essentially a part of the broader pharma industry focused on innovative biological medicines, whereas pharma includes both traditional chemical drugs and biological drugs. In short, all biopharmaceutical companies are pharmaceutical companies, but not all pharmaceutical companies are biopharmaceutical.
What is the difference between biopharmaceuticals and traditional drugs?
Biopharmaceuticals (biological drugs) and traditional drugs (chemical medicines) differ in how they are made, what they are made of, and how they behave:
- Origin and molecular size: Traditional drugs are small molecules produced through chemical synthesis in a lab, while biopharmaceuticals are larger and more complex substances derived from living organisms (e.g., proteins made of thousands of amino acids). Designing, characterizing, and producing a biopharmaceutical is much more complex and expensive than a simple drug. There’s also some variability between batches of biopharmaceuticals, as no two living cells produce proteins exactly the same way, unlike with simple chemical molecules. For example, aspirin (a traditional drug) is made through a well-defined chemical process always yielding the same molecule, while recombinant insulin (a biopharmaceutical) involves living cells and tight process control to maintain consistency.
- Production: Traditional drugs are synthesized by mixing chemical reagents. In contrast, biopharmaceuticals are “grown.” A human or animal gene is inserted into a microorganism or cell line, and those cells produce the drug. Then it must be purified from among many other biological components. For example, insulin was originally extracted from animal pancreases, but modern insulin is made by inserting the human insulin gene into bacteria, which produce it in fermentation tanks. This biological process is much more delicate and must be done under strict sterile conditions.
- Copies or generics: Due to their complexity, biopharmaceuticals cannot be copied exactly like chemical drugs. When the patent for a chemical drug expires, companies can make an identical copy called a generic. But when a biopharmaceutical’s patent expires, it’s impossible to make an exact molecular copy; instead, companies develop biosimilars, which are highly similar versions, not identical. Biosimilars must undergo additional studies to prove they perform like the original biopharmaceutical. In contrast, a chemical generic only needs to prove it contains the same molecule and absorbs similarly.
- Immunogenicity and administration: Since biopharmaceuticals are proteins or cells, they may trigger immune responses (the body may recognize them as foreign). This requires monitoring for allergies or resistance (anti-drug antibodies). Traditional drugs rarely provoke complex immune reactions. Also, administration routes differ: biopharmaceuticals are usually not given orally, as digestive enzymes would destroy them, they are administered by injection or intravenous infusion. Many traditional drugs come as tablets or capsules taken orally (e.g., antibiotics or painkillers). Additionally, biopharmaceuticals often require cold storage for stability (like vaccines), while chemical drugs are typically stable at room temperature.
In summary, biopharmaceuticals are much more complex in composition and manufacturing, are produced by living cells, and require special considerations for use. Traditional drugs are simpler chemical molecules, synthesized in labs, and can be copied exactly. Both have transformed medicine, each with its own advantages and challenges.
| Aspect | Biopharmaceuticals | Traditional Drugs |
|---|---|---|
| Origin & Molecular Size | Large, complex molecules (e.g., proteins) from living organisms; variability between batches. | Small, simple molecules made by chemical synthesis; consistent across batches. |
| Production | Grown using genetically modified cells; requires sterile cultivation and purification. | Synthesized by mixing chemical reagents in a lab. |
| Copies or Generics | Cannot be exactly copied; biosimilars are developed and require additional validation. | Can be exactly copied as generics; require only bioequivalence studies. |
| Immunogenicity & Administration | May trigger immune responses; administered by injection or infusion, not orally. | Rarely cause immune reactions; typically administered orally (tablets, capsules). |
| Storage | Often requires cold storage to maintain stability (e.g., vaccines). | Usually stable at room temperature. |
| Overall Complexity | High complexity in design, manufacturing, and regulation. | Lower complexity; simpler and cheaper to produce and regulate. |
What is considered a biopharmaceutical?
The term biopharmaceutical includes a wide variety of therapeutic products of biological origin. In general, any medicine whose active component comes from a living organism or from the biotechnological manipulation of one is considered a biopharmaceutical. Here are some representative examples:
- Vaccines: Biological preparations that stimulate immunity to prevent infectious diseases. For example, the hepatitis B vaccine (produced using recombinant DNA in yeast cells) or mRNA vaccines for COVID-19, which use genetic instructions to prompt our cells to produce a viral protein and trigger an immune response. Vaccines are considered biopharmaceuticals because they are obtained from attenuated or inactivated viruses, or through cell culture.
- Monoclonal antibodies: Lab-designed immune system proteins that bind specifically to a target (antigen). They are used in cancer treatments (e.g., trastuzumab for breast cancer), autoimmune diseases (e.g., adalimumab for rheumatoid arthritis), and other conditions. Since they originate from cells (typically genetically modified Chinese hamster ovary cells or similar), they fall under the biopharmaceutical category.
- Recombinant hormones and proteins: Many human hormones used as medicines are now biotechnologically produced. Classic examples: recombinant insulin for diabetes (previously obtained from pig pancreases, now produced in modified bacteria), erythropoietin (EPO) to treat anemia, or human growth hormone for growth disorders. These are molecules naturally produced by the body, but as medicines, they are manufactured using genetic engineering in living cells.
- Blood derivatives and human/animal-based products: This includes medicines obtained directly from tissues or biological fluids. Examples: plasma-derived clotting factors for hemophilia, immunoglobulins (polyclonal antibodies) from donors for immunodeficiencies, or even cell and tissue transplants (like bone marrow transplants or stem cell therapies). Historically, many of these came from animals or human donors. A curious example: insulin used to be extracted from animal pancreases, and antivenom serum is produced by immunizing horses to obtain antibodies against snake venom, a biological process. All of these are biopharmaceuticals because their active ingredients come from living organisms.
- Advanced gene and cell therapies: In one of the most innovative medical fields, gene therapies (introducing functional genetic material to correct diseases) and cell therapies (like CAR-T therapy for leukemia, where a patient’s T cells are genetically modified) are considered biopharmaceuticals. For instance, Luxturna is a gene therapy approved for a type of inherited blindness; it uses a modified virus to deliver a functional gene into a patient's eye. Although these are not “traditional” pills, they are regulated as biological drugs and therefore fall under the biopharmaceutical umbrella.
In summary, a biopharmaceutical is any medicine that uses biological or biotechnological agents as the basis for its therapeutic effect. From vaccines to monoclonal antibodies or modified cells, they all share a biological origin. This broad definition is supported by agencies like the EMA and FDA. For example, the European Medicines Agency (EMA) defines a biological medicine as one whose active substance is produced by or derived from a living organism.
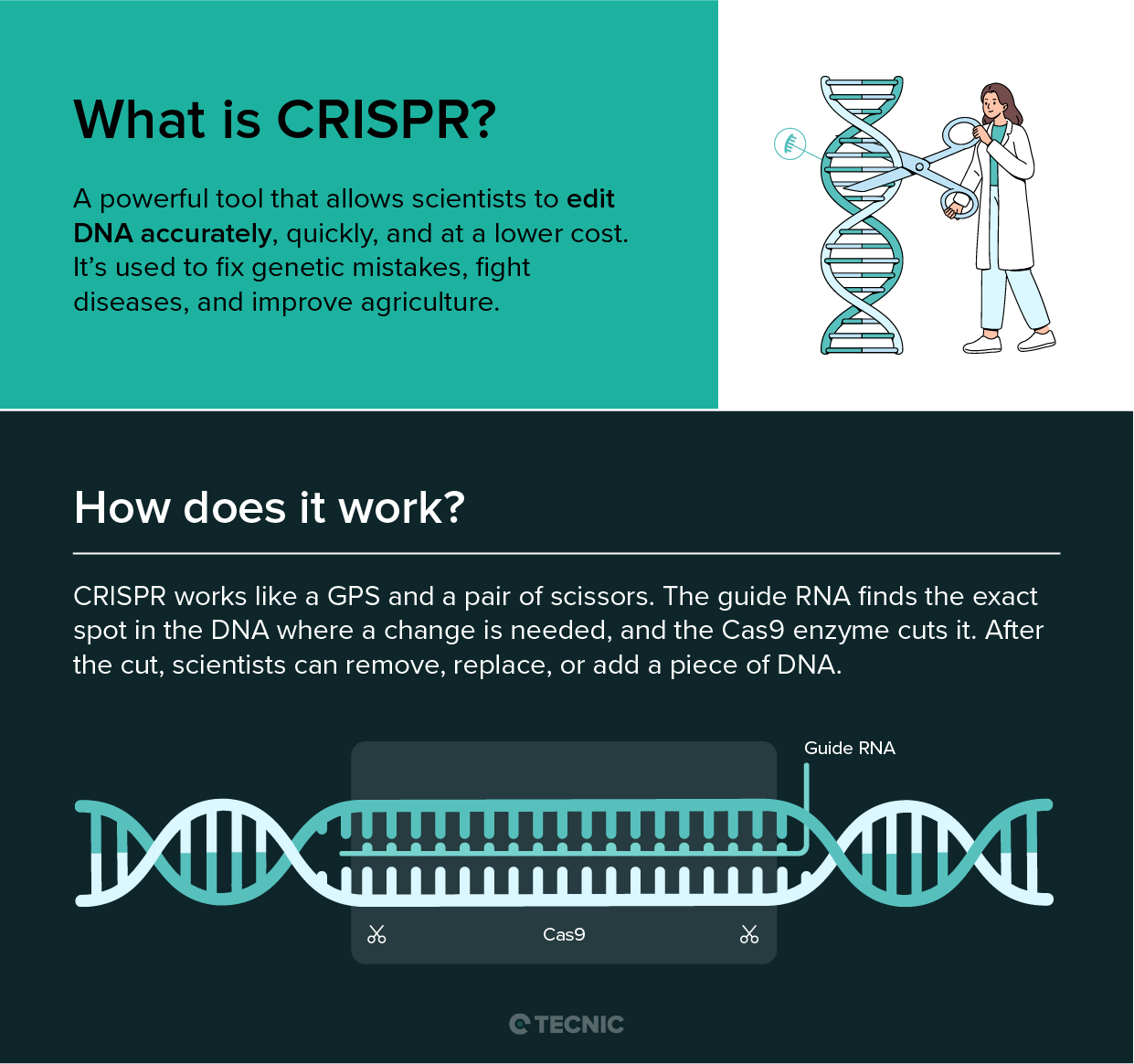
What are the four categories of biopharmaceutical products?
The main classification of biopharmaceuticals typically distinguishes four broad categories based on their origin and nature:
- Extracts from living organisms: These are the oldest biological products, obtained directly from living sources (human or animal) without genetic modification. They include blood components (blood, plasma, clotting factors), polyclonal antibodies from serum (e.g., antitoxins for venom), hormones or enzymes extracted from animal tissues, organ and tissue transplants, and even therapeutic uses of breast milk or fecal microbiota. These extracts were the first “biological medicines.” A classic example is insulin extracted from bovine or porcine pancreas before the advent of genetic engineering.
- Products obtained through recombinant DNA: These are modern biopharmaceuticals made using genetic biotechnology. This category includes most therapeutic proteins created by cloning human genes into microorganisms or cultured cells. Examples include:
- Recombinant hormones and cytokines: Such as human insulin, erythropoietin, growth hormone, interferons, and growth factors, produced in bacteria or other cells with inserted human genes.
- Monoclonal antibodies: Although also proteins, these are often highlighted due to their significance. They are produced using hybridoma technology or genetically modified cells that secrete a specific antibody (e.g., rituximab, infliximab).
- Fusion proteins and constructs: Such as soluble receptors fused to antibody fragments (e.g., etanercept, a TNF receptor fused to an antibody’s Fc portion).
All of these are manufactured in bioreactors using transgenic cells and represent a large share of today’s biopharmaceuticals.
- Vaccines: Vaccines form a standalone category due to their major public health role. Most vaccines are made by growing viruses, bacteria, or cells in special media, then inactivating or attenuating them, or extracting components (antigens) to trigger immunity. Recombinant vaccines also exist, only the relevant protein is produced in the lab (e.g., the hepatitis B vaccine uses yeast to produce the virus’s surface protein). Recently, nucleic acid vaccines (DNA or mRNA, like COVID-19 vaccines) have also emerged, and these are also considered biopharmaceuticals. In short, all vaccines, traditional or next-gen, are biopharmaceuticals, as their production relies on biological processes (cell culture, genetic engineering, etc.).
- Gene and cell therapies: This category includes treatments in which the therapeutic agent is genetic material or modified cells, rather than a conventional protein or molecule. In typical gene therapy, a modified viral vector is used to introduce a functional gene into a patient’s cells that carry a defective one. By manipulating the genome of viruses and cells, very unique “medicines” are created. This category also includes cell therapies, such as stem cell infusions or CAR-T treatments (genetically modified T cells from the patient). These advanced therapies are regulated as biopharmaceuticals by agencies and require rigorous evaluations.
In conclusion, biopharmaceuticals can be classified into traditional biological extracts, recombinant products (proteins/antibodies), vaccines, and gene/cell therapies. Each category reflects a different mode of production and type of therapeutic agent, but they all share the common feature of being derived from biology. This classification helps us understand the wide range of biopharmaceuticals available, from the most “classic” (like blood or insulin) to the most innovative (like gene vectors or modified cells).

How are biopharmaceuticals developed and produced?
Creating a biopharmaceutical is a complex process that combines biology, engineering, and strict quality control. The main stages are:
- Research and genetic design: First, the desired therapeutic molecule is identified (e.g., a gene encoding a beneficial human protein or a specific antibody). Scientists isolate or synthesize the gene and insert it into a vector (like a DNA plasmid). This vector is used to introduce the gene into host cells (bacteria, yeast, or mammalian cells). Essentially, a production organism is “designed” through genetic modification. For example, to produce human insulin, the insulin gene is inserted into E. coli bacteria.
- Cell culture (bioproduction): Once the genetically modified cell line is created to produce the biopharmaceutical, it is cultivated at scale. Bioreactors, special tanks, are used to maintain optimal conditions for the cells to grow and produce the desired protein or product. These tanks range from small lab-scale units (a few liters) to industrial facilities holding thousands of liters.
- Recovery and purification: After cultivation, the culture broth contains a complex mix (cells, nutrients, waste products, and the produced biopharmaceutical). The next step is to extract and purify the biopharmaceutical. Advanced separation techniques are used: filtration, chromatography (which separates molecules by chemical properties), centrifugation, etc. The goal is to isolate the active protein or product at high purity, removing contaminants (such as cell debris, DNA, or other proteins). This is critical, biopharmaceuticals require extreme purity, as even minimal impurities can cause adverse reactions in patients. For monoclonal antibodies, multiple chromatography steps are performed to achieve near 100% purity. For cell therapies, “purification” might mean selecting and concentrating the desired cells while removing non-viable ones.
- Formulation and quality control: Once purified, the active ingredient is formulated into a suitable pharmaceutical form (e.g., lyophilized and later reconstituted, or mixed with stabilizers). Throughout the process, stringent quality controls are in place. The identity of the molecule is confirmed (ensuring it is the correct protein with the proper 3D structure), its biological activity is verified, its purity is measured, and potential contaminants (microbial, viral, endotoxins, residual DNA, etc.) are checked. Due to their complexity, biopharmaceuticals undergo far more extensive testing than chemical drugs.
- Clinical trials and regulatory approval: Alongside pilot-scale production, the biopharmaceutical must demonstrate safety and efficacy in human clinical trials. These usually occur in phases (I, II, III), like with any drug. However, additional studies may be required for biopharmaceuticals, such as immunogenicity assessments (checking for unwanted immune responses) or comparability studies if manufacturing changes occur. Finally, regulatory agencies (FDA in the U.S., EMA in Europe, etc.) evaluate all data. Biopharmaceuticals are subject to strict regulations: manufacturing facilities must comply with Good Manufacturing Practices (GMP), and any process changes must be reported.
Once a biopharmaceutical passes these tests, it is approved for marketing. Even after approval, it continues to be monitored (pharmacovigilance), and each batch undergoes control tests before distribution. A key point: due to the complexity of production, biopharmaceuticals tend to be expensive, though prices tend to drop over time with the arrival of biosimilars.

Conclusion
Biopharmaceuticals represent the intersection of biology and pharmacy. We’ve seen what they are, how they are classified, and how they differ from traditional drugs. We’ve also explored examples (from insulin and vaccines to monoclonal antibodies) and summarized how they are taken from the lab to the patient via cell culture and regulatory processes. Understanding these concepts helps us appreciate how advances in genetics and biotechnology translate into new medical treatments. Biopharmaceuticals are already part of our lives, and in the future, we’ll likely see even more innovative biological therapies tackling diseases once thought incurable.
Frequently Asked Questions (FAQ)
It’s a drug produced using living cells or organisms, usually proteins or nucleic acids.
They’re biologically derived, while traditional drugs are chemically synthesized.
They treat diseases like cancer, autoimmune disorders, and genetic conditions.
Through complex processes like cell culture, fermentation, purification, and formulation.
Insulin, erythropoietin, monoclonal antibodies, and mRNA vaccines.
References
AIMPLAS – Instituto Tecnológico del Plástico. (s. f.). Bioplásticos: tipos, aplicaciones y perspectivas.
European Bioplastics. (2023). Bioplastics market data 2023.
Greenpeace. (2020). Decepción biodegradable: el mito del bioplástico.
National Geographic. (2018). Are bioplastics really better for the environment?
Friends of the Earth Europe. (2020). The truth about bioplastics.
European Commission. (2018). A European strategy for plastics in a circular economy.







