What is cell therapy?
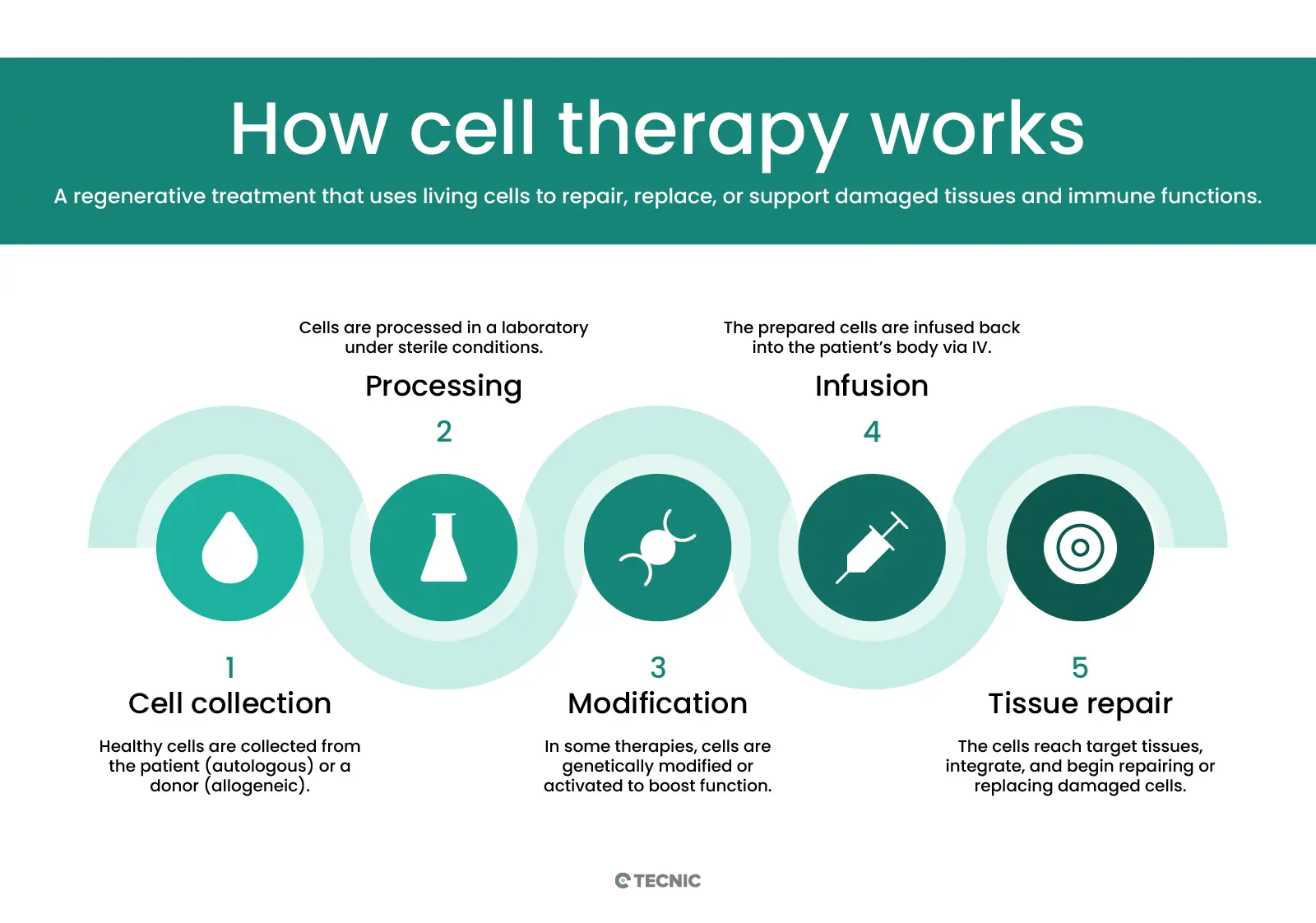
Cell therapy involves giving patients living cells to replace or repair diseased tissues. The American Society of Gene & Cell Therapy (ASGCT) defines cell therapy as “the transfer of a specific cell type(s) into a person to treat or prevent a disease”. In practice, a doctor may infuse stem cells or immune cells (for example, blood-forming stem cells or engineered T cells) into the patient’s body. These cells travel to damaged tissues, multiply, and restore function. The goal is to help the body regrow or heal the damaged cells. In other words, cell therapy is a form of regenerative medicine that aims “to grow, replace, or repair damaged tissue”. For example, mesenchymal stem cells have been injected to help heal inflamed or injured joints, and blood stem cells are transplanted to rebuild the bone marrow after cancer treatment.
How does cell therapy work?
Cell therapy works by transferring living cells into the patient’s body. The cells can come from the patient themself or from a donor. In autologous therapy, doctors collect a patient’s own cells (such as bone marrow or fat stem cells), modify or expand them in the lab, and then return them to the patient. Because they are the patient’s own cells, this lowers the risk of immune rejection. In allogeneic therapy, the cells come from another person (a matched donor).
For example, a healthy donor’s bone-marrow stem cells can be given to a patient who needs a new blood system. Before receiving any cell infusion, patients often undergo conditioning (chemotherapy or radiation) to suppress their immune system or clear space in a tissue (like bone marrow). After infusion, the new cells travel to the target organ, engraft (take root), and begin growing. Over weeks to months, they multiply and differentiate into needed cell types. For instance, donated bone marrow stem cells will divide and become healthy blood cells, restoring the patient’s blood-forming system.
What are autologous and allogeneic cell therapies?
Autologous and allogeneic refer to the cell source. Autologous cell therapy uses the patient’s own cells. Doctors harvest cells from the patient (for example, stem cells from bone marrow), manipulate them if needed (perhaps adding a gene), then infuse them back. Because these are “self” cells, there is little risk the immune system will reject them. Allogeneic cell therapy uses cells from a donor. Donor cells must be carefully matched to avoid immune attack. Allogeneic products are often “off-the-shelf” therapies (ready-made), since they can be prepared in advance and used when needed. The ASGCT notes that “allo” means other, emphasizing donor origin. In practice, many stem cell transplants (like for leukemia) are allogeneic: healthy donor marrow gives new blood-building cells. CAR-T cell therapies (for cancer) are usually autologous: the patient’s own T cells are collected, engineered to attack cancer, and then reinfused.
What diseases can cell therapy treat?
Today most approved cellular therapies target blood and immune system disorders. For example, hematopoietic (blood-forming) stem cell transplants are a standard therapy for leukemia, lymphoma, multiple myeloma, and other bone-marrow cancers. CAR T-cell therapies (genetically modified T cells) can induce remissions in certain blood cancers (acute lymphoblastic leukemia, lymphoma). Beyond cancer, stem cell transplants treat immunodeficiency syndromes and genetic blood disorders (like thalassemia).
Research is expanding into many other diseases. Because stem cells can become many tissues, scientists are testing cell therapies for heart disease, spinal cord injury, diabetes, and joint (cartilage) damage. For example, small trials have injected cardiac stem cells after a heart attack to help regenerate muscle, or transplanted stem-cell-derived pancreatic islet cells for diabetes. Early studies show promise: Some companies report that stem cells can be guided to “regenerate and repair tissues that have been damaged or affected by disease”. People who might benefit include those with degenerative conditions like Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and osteoarthritis. In all cases, the aim is to replace the non-functioning cells with healthy new ones so that organs and tissues can heal.
How does cell therapy regenerate tissue?
In cell therapy, the injected or infused cells actively rebuild the damaged tissues. Stem cells have the special ability to become multiple cell types. Once administered, they differentiate into the needed cells and integrate into the organ. For instance, a stem cell injected into a patient’s knee joint may turn into cartilage cells and help repair a worn joint. Some clinics explain that researchers can “generate healthy cells to replace cells affected by disease (regenerative medicine)” using stem cells. In animal studies, scientists have even reprogrammed skin cells into heart muscle cells and injected them into heart-failure models – these new cells improved heart function and survival. In short, cellular therapy works like a living repair kit: millions of cells are delivered where they’re needed, then grow and produce the proteins and structures that rebuild the tissue.
What are the risks of cell therapy?
Cell therapies can have serious side effects. Autologous therapies (using self cells) mostly avoid rejection, but allogeneic (donor) cells carry the risk of graft-versus-host disease (GVHD). In GVHD, the donor’s immune cells attack the patient’s normal tissues, which can cause rash, liver damage, gut injury and even be life-threatening. Cancer patients receiving cell therapies often undergo strong immunosuppression first; this raises infection risk. The ASGCT warns that pre-treatment with chemotherapy can “increase infection risk… and can be quite hard on the body”.
Another major risk with immune-based therapies is an overwhelming immune reaction. CAR T-cell treatments, for example, can trigger cytokine release syndrome – a dangerous “storm” of immune signals. Symptoms include high fever, low blood pressure, and multi-organ toxicity. ASGCT notes that “too many cytokines can result in fever, trouble breathing and can be life-threatening”. Hospitals giving CAR T also monitor for neurologic toxicities (confusion, seizures).
Importantly, because cellular therapy is a new field, long-term effects are still under study. Clinical trials include rigorous informed consent so patients know potential risks. Physicians follow strict guidelines to watch for complications. If any dangerous side effects appear, trials can be paused. Overall, while many cell therapies have been life-saving, they can also trigger severe immune or toxic reactions.
How much does cell therapy cost?
Advanced cell therapies are very expensive. CAR T-cell therapies, for example, often cost several hundred thousand dollars per course. Studies have found that the total medical cost of a CAR T treatment (including hospital care) averages around $450,000–$500,000. In patients with severe complications, costs can exceed $500,000. Bone marrow stem cell transplants (another form of cell therapy) also run high, they can range from tens of thousands to over $200,000 depending on whether it’s autologous or allogeneic. These prices reflect the complex preparation (cell collection, genetic modification, specialized care) and the intensive hospital stays often needed. Insurance and healthcare systems are still figuring out how to cover such therapies.
What is gene therapy?
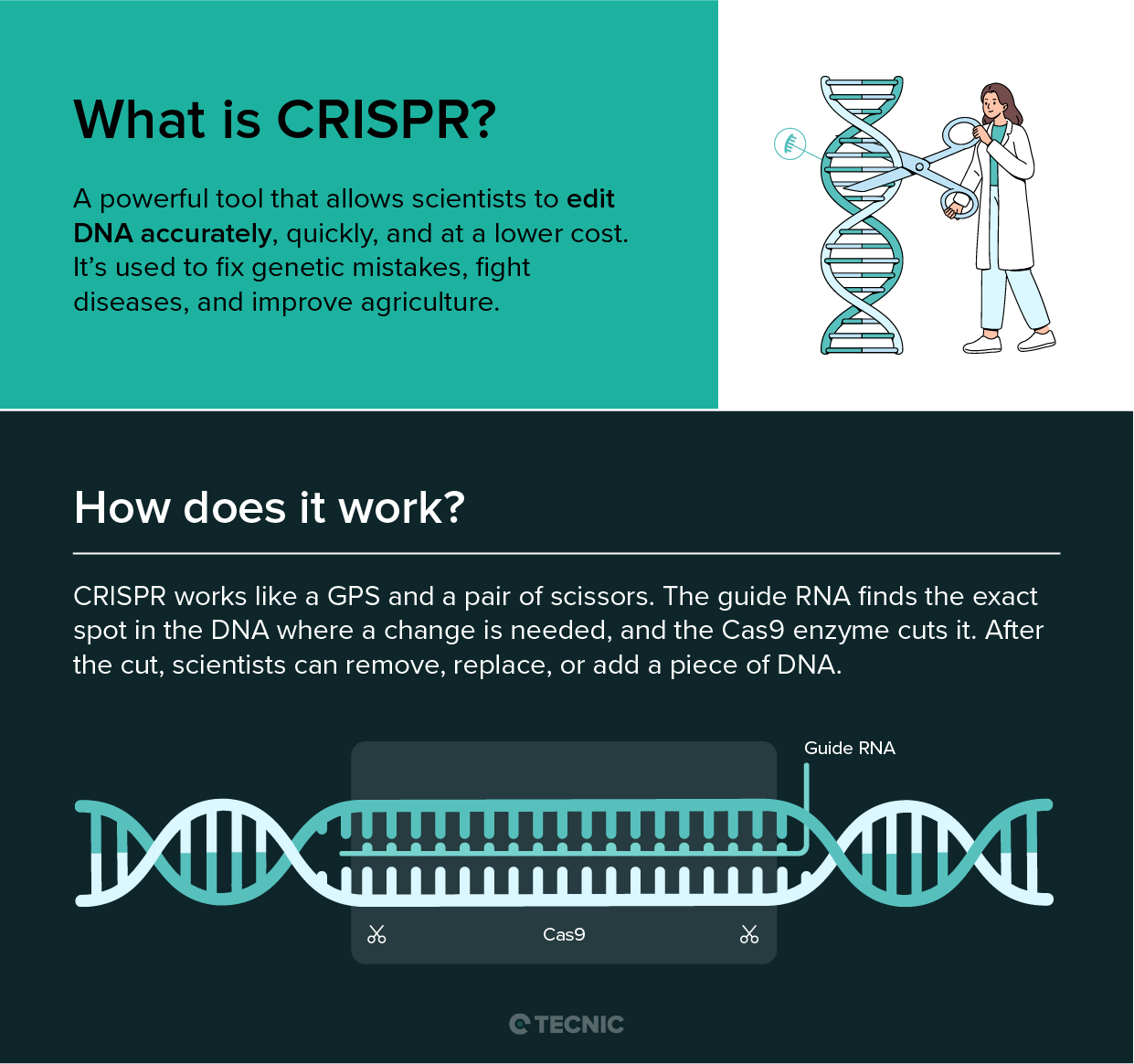
Gene therapy is a technique that treats disease by fixing or manipulating a person’s genes. The National Library of Medicine defines gene therapy as a “medical approach that treats or prevents disease by correcting the underlying genetic problem”. In practice, gene therapy introduces new genetic instructions into a patient’s cells. For example, a healthy copy of a gene can be added to a patient who has a faulty version. Early genetic therapies (called gene addition) aimed to introduce a working gene to stand in for a mutant gene. Newer methods use gene editing: molecular tools like CRISPR-Cas9 can cut, replace, or switch off specific parts of DNA to correct mutations. In any case, the goal is to change the patient’s genetic code so that cells make the proper proteins.
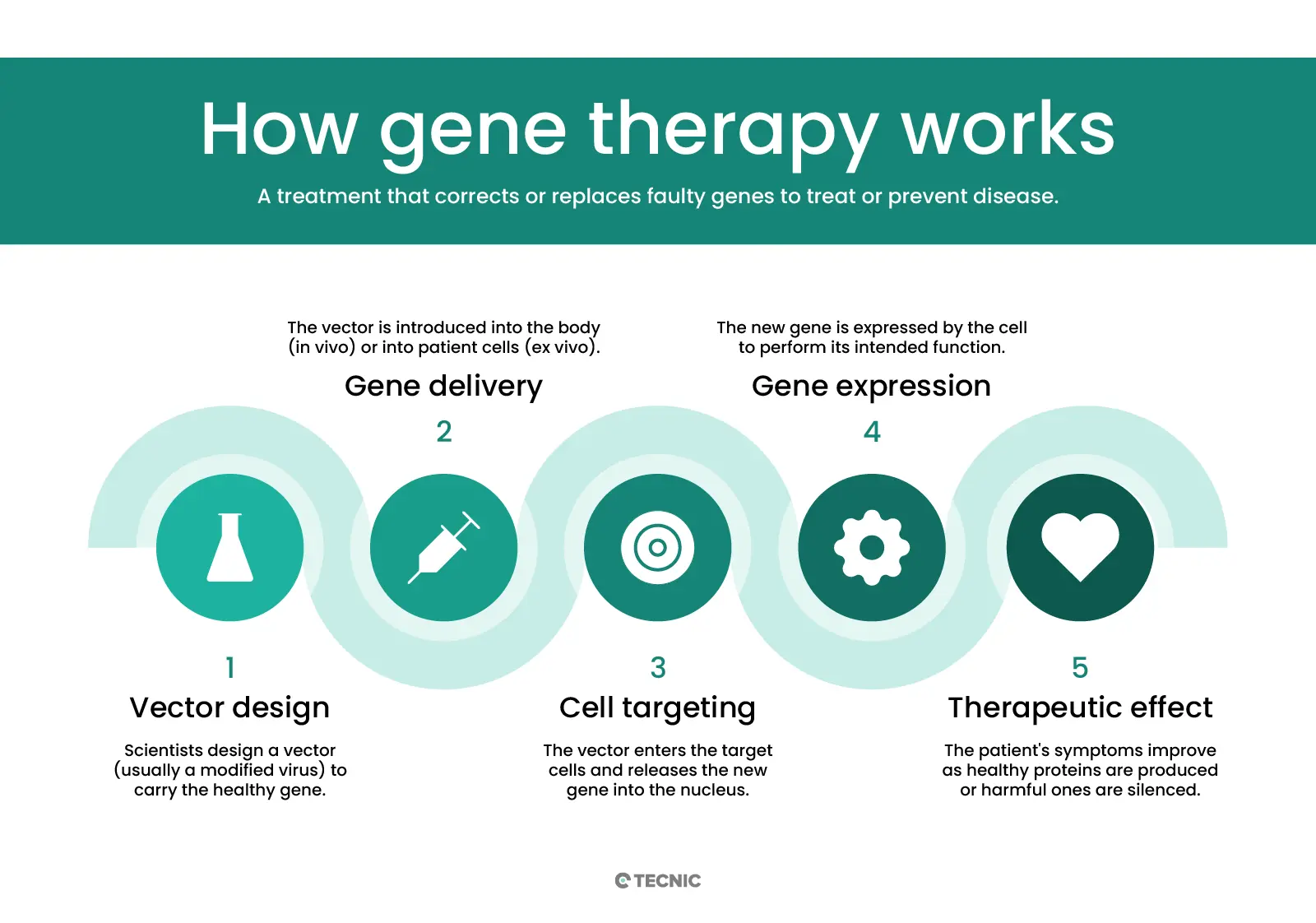
How does gene therapy work?
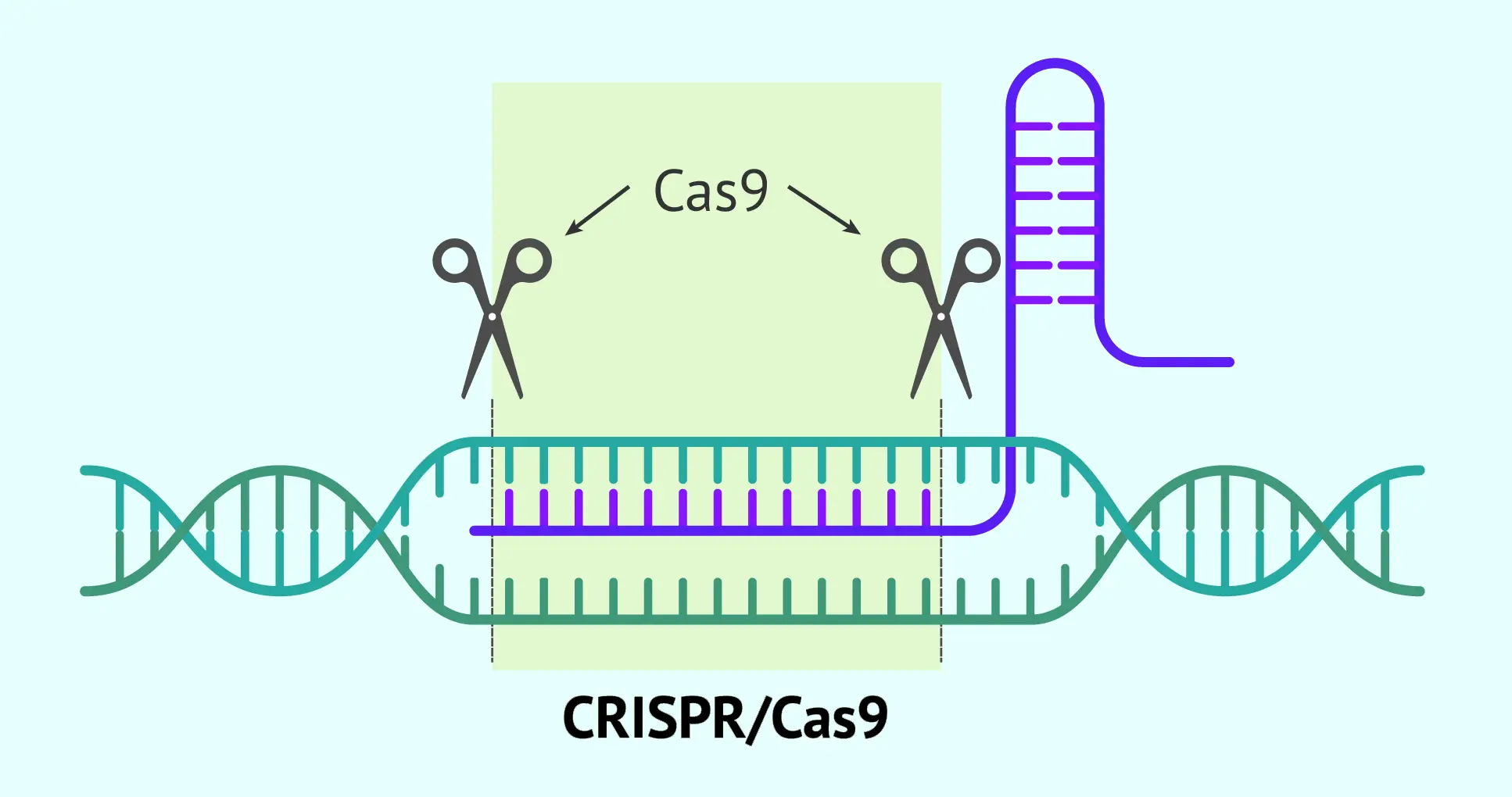
Gene therapy delivers genetic material into cells using a carrier called a vector. A vector is like a microscopic delivery truck: it carries new DNA or RNA into the target cells. Viral vectors are most common, because viruses naturally inject DNA into cells. Scientists take a harmless virus (e.g. an adeno-associated virus, AAV, or a lentivirus) and remove its disease-causing genes. The virus’ shell can still enter cells, so it safely carries the therapeutic gene inside. For instance, an AAV vector injected into a patient’s bloodstream will travel to specific cells and insert the new gene. Non-viral methods (like lipid nanoparticles or CRISPR machinery) are also being studied to avoid viral complications.
Once inside, the vector delivers the new gene to the cell’s nucleus. The cell then uses that gene to make healthy proteins. In in vivo gene therapy, this process happens inside the patient’s body – vectors are given by IV infusion or injection into the target organ. In ex vivo gene therapy, doctors remove some of the patient’s cells first, modify them in the lab, and then put them back. For example, bone marrow stem cells might be taken out, a functional gene inserted using a viral vector, and then returned to the patient’s blood stream. This is actually similar to cell therapy: it infuses living cells back into the patient, but those cells carry a new gene.
What are types of gene therapy?
Gene therapies can be classified by how and where they act. First, there is somatic vs germline genetic therapy. Somatic gene therapy affects only the patient’s body (any cell except eggs or sperm). These changes are not passed to future generations. Germline therapy would alter sperm, eggs, or embryos so that the change is inheritable by the patient’s children. Germline editing is highly controversial and currently illegal in most countries. Research so far focuses on somatic approaches.
Second, gene therapy can be in vivo vs ex vivo, as described above. In vivo means the genetic material is delivered directly inside the body (for example, an eye injection of a viral vector for an inherited blindness). Ex vivo means cells are modified outside the body. Both approaches are used in the clinic. For example, many of the blood disorder gene therapies use ex vivo methods (edit the patient’s blood stem cells outside and return them). Researchers also distinguish based on technology: traditional gene therapy adds or replaces genes, while gene editing (like CRISPR) precisely cuts or alters existing genes.
What delivery methods are used in gene therapy?
The most common delivery methods are viral vectors. The three viral vectors widely used in approved therapies are adeno-associated virus (AAV), lentivirus, and adenovirus. AAV is popular because it produces mild immune reactions and can deliver genes to many cell types. Lentivirus (derived from HIV) is used especially in ex vivo therapies (it can insert larger genes into dividing cells). Researchers remove most of the virus DNA, keeping only the “shell” and insertion machinery. For example, AAV is used to deliver the RPE65 gene in Luxturna (an eye gene therapy).
Non-viral methods are also under active research. These include plasmid DNA injections, lipid nanoparticles, and direct RNA delivery. Physical methods like electroporation (electric pulses) or chemical carriers can ferry genes into cells without viruses.
A notable new technology is CRISPR-Cas9 gene editing. Instead of adding whole genes, CRISPR delivers molecular “scissors” that cut DNA at precise spots. Doctors can design CRISPR tools to remove a disease-causing mutation or turn genes on/off. Early CRISPR-based therapies are in trials for blood diseases and cancer. So far, approved therapies mostly use viral vectors to add genes, but CRISPR and other gene-editing methods promise more precise fixes in the near future.
What diseases can gene therapy treat?
Gene therapy is especially suited for genetic disorders where a single gene defect causes disease. To date, only a small number of gene therapies are FDA-approved. For example, Luxturna treats an inherited blindness (Leber congenital amaurosis) by adding a working copy of the RPE65 gene. Zolgensma is approved for spinal muscular atrophy (a fatal infant disease) and replaces the missing SMN1 gene. Hemgenix treats hemophilia B by providing the clotting factor IX gene; it costs about $3.5 million for a one-time infusion.
Other rare diseases with ongoing gene therapy trials include Duchenne muscular dystrophy, severe combined immunodeficiency, sickle cell disease (via gene-edited blood cells), and metabolic disorders like Gaucher or Fabry disease. Researchers are also exploring gene therapy for some cancers: for example, introducing “suicide genes” or immune-enhancing genes into tumors. In summary, genetic therapy can potentially target any disease caused by a faulty gene. So far it’s been used for inherited diseases of the eye, muscle, blood, and immune system, among others.
What are the risks of gene therapy?
Because gene therapy involves altering DNA, there are important safety concerns. Early genetic therapy trials (over 30 years ago) revealed that introducing genes could cause severe immune reactions or even trigger cancer. For instance, patients in a trial developed leukemia after gene insertion activated other oncogenes. These experiences taught researchers how to improve safety (using better vectors, tighter control of gene insertion).
Current risks include immune responses to the delivery vector. Even though vectors are “disarmed,” the body may see them as foreign. This can cause inflammation or destroy the therapeutic vector before it works. In extreme cases, a strong immune reaction could be life-threatening (as happened in an early adenovirus trial). Another risk is off-target effects. If a vector inserts DNA in the wrong place, it could disrupt another gene or regulatory region. Modern trials monitor patients long-term to watch for delayed cancers or other issues.
Gene editing adds another layer of risk. CRISPR cuts the genome at targeted sites, but it can sometimes cut at similar sequences (off-target cleavage). Unintended edits might inactivate an important gene or create a harmful mutation. Scientists must carefully validate that edits are precise.
Finally, ethical and genetic risks surround germline changes. Because altering a fertilized embryo’s DNA would affect all future cells, it is highly controversial and banned by regulators. All current clinical gene therapies change only somatic cells (no heritable changes).
Regulatory agencies (FDA, NIH) closely oversee gene therapy trials. They require multi-phase clinical testing (Phase I safety, Phase II efficacy, etc.). Informed consent, independent ethics review boards, and long-term follow-up are mandatory. Although no therapy is risk-free, advances have greatly improved safety, and several gene therapies have now been approved for patient use.
How much does gene therapy cost?
Gene therapies tend to be extremely expensive, often one-time treatments with multi-million dollar price tags. For example, when Luxturna was approved in 2017, it cost $425,000 per eye. The spinal muscular atrophy therapy Zolgensma costs roughly $2.1 million for a single infusion. Hemgenix, for hemophilia B, was priced at about $3.5 million – currently among the most expensive drugs in the world. These high costs reflect the complex development and the fact that one dose can provide lasting benefit for years or decades.
By comparison, traditional medications cost far less. However, patients with severe genetic diseases sometimes require lifelong treatments (infusions every few weeks) that add up. Gene therapy’s one-time approach has led companies to charge premium prices. Insurers and health systems are still negotiating ways to pay for these “curative” therapies without bankrupting patients or public funds.
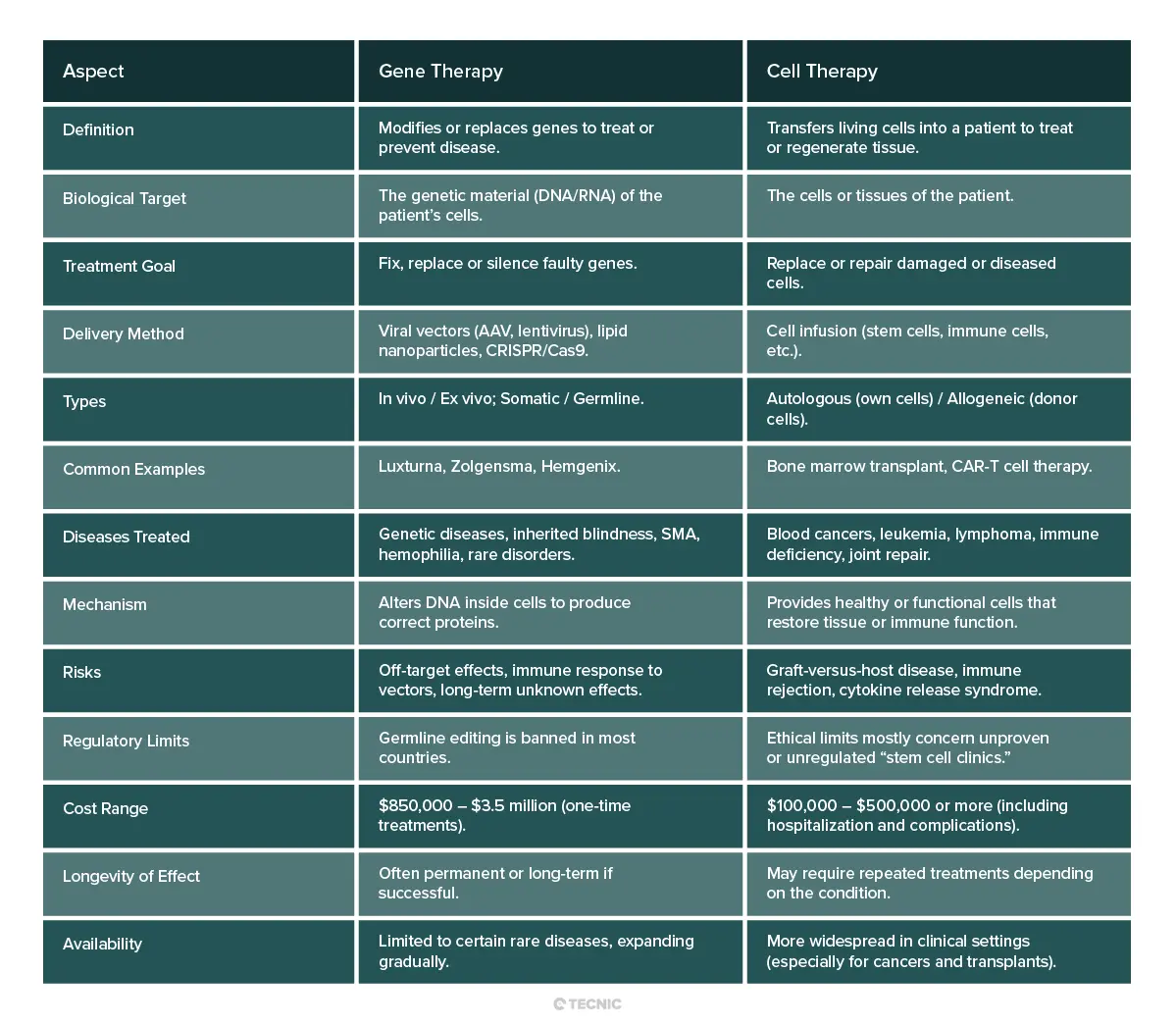
What is the difference between gene therapy and cell therapy?
At a basic level, gene therapy and cell therapy are different strategies. Gene therapy works by transferring genetic material into a patient’s cells (often using viral vectors). Cell therapy works by transferring living cells themselves into the patient. In gene therapy, the therapy is the new gene or editing construct. In cell therapy, the therapy is the population of cells (which may or may not have been genetically modified).
There are overlaps. For example, CAR T-cell therapy is a hybrid: a patient’s T cells (cell therapy) are taken out, genetically modified to carry a cancer-fighting gene, and then reinfused, so it is sometimes called “cell-based gene therapy.” But in general, genetic therapy aims to fix the DNA defect, whereas cell therapy aims to replace or boost cell function.
Which is better gene therapy or cell therapy?
There is no simple answer; it depends on the disease and patient. For inherited genetic disorders (like hemophilia, retinal disease, or enzyme deficiencies), gene therapy directly addresses the root cause by adding a correct gene. It can potentially cure the condition at its source. For conditions caused by cell loss or dysfunction (such as leukemia or organ damage), cell therapy may be more effective. For example, leukemia is often treated with a new immune system via stem cell transplant or CAR T cells, which is not something genetic therapy alone could do.
In some cases, both approaches might work. For a patient with a genetic blood disorder, doctors might either add a healthy gene into blood cells (gene therapy) or give the patient a stem cell transplant (cell therapy). Which is “better” depends on factors like how well the patient tolerates a transplant vs a gene treatment, the specific genetics of the disease, and cost/availability. In short, gene and cell therapies are complementary tools. Neither is inherently superior – physicians choose the one that best fits the medical context.
What are the benefits and risks of gene therapy vs cell therapy?
Benefits: Both therapies offer the promise of long-lasting cures rather than just symptom control. Gene therapy can fix a malfunctioning gene, potentially restoring normal cell function permanently. Cell therapy can regenerate damaged tissues or empower the immune system. For example, gene therapy has already cured some children of diseases previously fatal (like severe combined immunodeficiency), and cell therapy (like CAR T) has achieved remissions in patients with otherwise untreatable cancers. These treatments open possibilities far beyond what traditional drugs can do.
Risks: As discussed, both have unique dangers. Gene therapy risks include immune reactions to the vector and unintended DNA changes. Cell therapy risks include graft reactions and severe inflammatory side effects. A patient undergoing either must be closely monitored. Additionally, because both approaches are relatively new, long-term consequences are not fully known. Clinical trials continue to collect data on durability and late effects.
Cost is also a risk factor: these therapies are so expensive that access may be limited. This raises questions of equity and affordability. As the National Institutes of Health notes, a concern is whether “the high costs of genetic therapy make it available only to the wealthy”. Ultimately, patients and families must weigh the potential for a cure against the potential side effects and financial/ethical considerations.
What ethical concerns are associated with gene therapy and cell therapy?
Several ethical issues arise. For gene therapy, the main concerns involve genetic alteration of the body’s core blueprint. Most researchers agree it is ethical to treat a patient’s somatic cells. But germline editing (making heritable changes) is highly contentious. Editing an embryo or sperm/egg could affect future generations who cannot consent, and it carries unknown risks. In fact, germline gene editing is currently banned in the U.S., Europe, and many countries. There are also questions about “designer genetics” – using genetic therapy for enhancement (height, intelligence, etc.) rather than disease prevention. Society must decide where to draw the line.
For cell therapy, key ethical concerns include safety and fraud. Because stem cell therapies are so promising, some clinics sell unproven treatments directly to patients without proper trials. The ASGCT warns that “some unethical providers… offer cell therapy products that have not [been] tested to see if they work, and may be dangerous”. This has led to patient harm in some cases. Legitimate cell therapies must go through clinical trials and regulatory review. Patients are advised to be cautious of “stem cell clinics” that claim miracle cures.
Both fields also face questions of justice and access. If these advanced therapies remain extremely expensive, will only wealthy individuals benefit? Will insurance cover them? These treatments could make society less accepting of people with genetic conditions if everyone expects “perfect” genes.
In summary, gene and cell therapies bring tremendous hope but also require careful ethical oversight. Ongoing dialogues among scientists, ethicists, and the public aim to ensure these technologies are used safely and fairly.
Conclusion: A new era of medicine, powered by biotechnology
Cell therapy and gene therapy represent two of the most promising frontiers in modern medicine. By treating diseases at their root, whether by restoring damaged tissues or correcting faulty genetic instructions, these therapies are reshaping how we approach cancer, rare disorders, and regenerative care. While each therapy has its specific mechanisms, risks, and applications, both share the potential to deliver lasting, transformative results where traditional treatments fall short.
As the field advances, the integration of advanced bioprocessing, automation, and single-use technologies becomes increasingly essential. This is where TECNIC plays a vital role. With a global presence and a portfolio that includes modular bioreactors, perfusion platforms, TFF systems, and single-use flowkits and bags, TECNIC provides the infrastructure that enables safe, scalable, and efficient development of cell and gene therapies.
From early-stage R&D to commercial-scale production, TECNIC supports biopharmaceutical companies and research institutions in bringing life-changing therapies to patients, faster, safer, and with full process control.

Frequently Asked Questions (FAQ)
Gene therapy modifies genetic material to treat or prevent disease, while cell therapy uses living cells to repair or replace damaged tissues or cells.
Not exactly. Stem cell therapies are a type of cell therapy, while gene therapy involves genetic modification. Some treatments combine both approaches.
Gene therapy is a medical treatment that involves modifying or replacing faulty genes inside the body’s cells to treat or prevent diseases, especially genetic disorders.
Cell therapy is a technique where live, healthy cells are introduced into a patient’s body to repair or replace damaged tissue, regenerate function, or strengthen the immune system.
They are generally safe under medical supervision, but like all treatments, they carry risks depending on the method, patient condition, and regulatory oversight.
Cell therapy is used for cancers (like CAR-T), immune disorders, orthopedic injuries, and degenerative diseases, among others.
Gene addition, gene editing (e.g., CRISPR), and gene silencing are common types of gene therapy used to treat genetic disorders and cancers.
References
-
American Society of Gene & Cell Therapy. (n.d.). Gene Therapy Basics.
-
American Society of Gene & Cell Therapy. (n.d.). Cell Therapy Basics.
-
National Human Genome Research Institute. (2022). What are the Ethical Concerns of Genome Editing?
-
U.S. Food and Drug Administration. (n.d.). Approved Cellular and Gene Therapy Products.
-
International Society for Stem Cell Research. (n.d.). The ISSCR Guide to Stem Cell Treatments.
-
Nature Biotechnology. (2023). CRISPR-Cas 9 genome.
-
ScienceDirect. (2021). Cell and gene therapy investment.